
Itla-okla (Tillandsia usneoides) fibre temper in Pre-Columbian ceramics
By Joeri Kaal (Pyrolyscience) and Zackary Gilmore (Department of Anthropology, Rollins College, USA)

Itla-okla, which means “tree hair”, was the Indian name of the epiphyte Tillandsia usneoides, also known as “Spanish beard” or “Spanish moss”. Pre-Columbian societies of the Late Archaic period (Orange and Stallings traditions; 3000-1800 B.C.) used these epiphytes as an organic temper in the production of pottery. In collaboration with Zackary Gilmore (Rollins College, Florida, USA), Pyrolyscience studied archaeological sherds of this fiber-tempered (FT) ware in order to identify (other) organic materials used for their fabrication and assess the production conditions of this extraordinary pottery (Gilmore, 2015).
Materials and Methods
The materials analyzed are (1) ceramics, ball-milled to powder and treated with HF to eliminate oxides, (2) isolated charred fiber materials, scraped from the archaeological sherds and (3) Spanish moss, both uncharred and charred in a temperature series between 300 and 600 ºC. The analysis of charred “moss” (it is neither a moss nor a lichen, but an angiosperm from the Bromeliaceae family) was thought to enable the estimation of the firing temperature (in terms of muffle furnace equivalent temperature, TMFE) of the isolated fibres and whole ceramic fragments. HF treatment was performed to eliminate reactive minerals. Pyrolysis-GC-MS was performed at 750 ºC to stimulate fragmentation of thermoresistant components such as charred organic matter (Black Carbon) (Kaal et al., 2009). Extant moss was double-wrapped in aluminum foil to create oxygen-limited conditions and charred between 300 and 600 ºC (e.g. Turney et al., 2006).
Results and Discussion
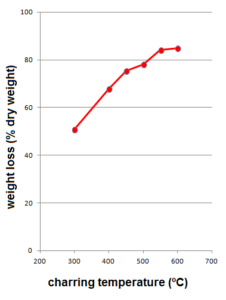
First we’ll discuss the molecular chemistry of the plant and it’s experimentally created charred equivalents. The loss of weight of the sample of fresh Itla-okla as induced by charring in the muffle furnace shows a typical increase from low to high temperatures: 50 % at TMFE=300 ºC towards 85 % at TMFE=600 ºC (Figure 1). This is known to reflect dehydration and condensation reaction as the temperature rises.
With Py-GC-MS, the uncharred sample (feedstock) is prolific of many polysaccharide products (acetic acid, furans, cyclopentenones, pyranones), lignin products (4-vinylphenol, guaiacols, syringol) and some aliphatic products such as phytadienes (from chlorophyll) and fatty acids. With THM-GC-MS, the main peaks are fatty acid methyl esters (C16, C18, C24-C28), mid-chain methoxylated C16 fatty acid methyl esters (from cutin in cuticula) and the lignin products P18 (p-coumaric acid methyl ester), G18 (ferulic acid methyl ester). These results represent the first molecular screening with Py-GC-MS and THM-GC-MS of Tillandsia usneoides feedstock.
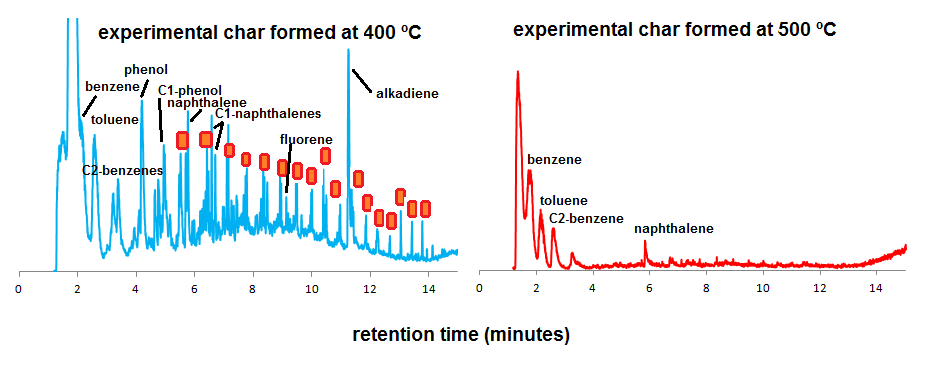
With increasing TMFE, the peak intensities (Py-GC-MS only) of these polysaccharide and lignin products decrease and those of monocyclic and polycyclic aromatic hydrocarbons (MAHs/PAHs) increase, which is a feature that has been observed in many kinds of feedstock materials of which experimental “thermosequences” were analyzed by Py-GC-MS. There is an intermediate range of thermal modification as recognized from Py-GC-MS fingerprints between 300 and 400 ºC with abundance of aliphatic products (alkanes, alkenes) and phenols (degraded lignin). The abundance of these phenols and aliphatic products (n-alkanes and n-alkenes from aliphatic biopolymers such as cutin) is high in the samples produced at 300 and 400 ºCMFE but not at 500 ºC and higher (here, the dominance of MAHs and PAHs is very strong) (Figure 2). These results are in agreement with previous studies of plant-derived chars.
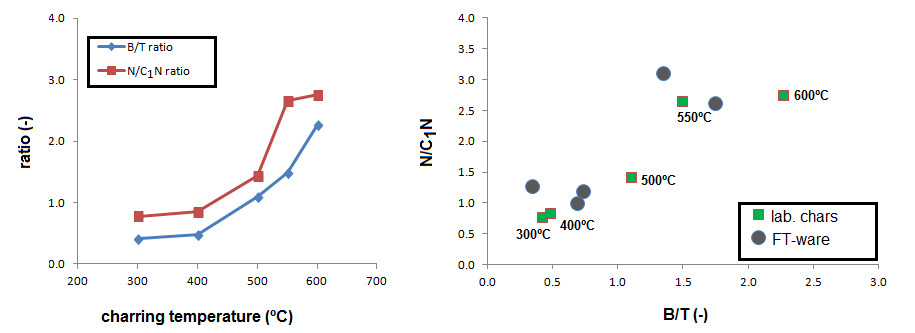
The relative abundances of benzene and toluene (B/T ratio) and naphthalene and methylnaphthalens (N/C1N ratio) has been used as an indicator of charring intensity. The graph below shows how these parameters increase with TMFE in the experimental graph, especially between 400 and 550 ºC. These trends can be used to compare with the fibres and whole sherds from the FT ceramics. The figure to the right shows the B/T – N/C1N plot of the laboratory chars and the isolated fibers, and shows that three of the fibers plot in the low-thermal impact range (<500 ºCMFE) whereas the other two plot in the high temperature range (450–600 ºCMFE). For the whole sherds, we could not establish B/T due to the existence of double peaks (implying a combination of volatiles and polymeric sources) and the methylnaphthalenes could not be reliably quantified due to low peak intensities.
This information can now be used to interpret the Py-GC-MS fingerprints of the fibers extracted from sherds and whole sherd samples (Figure 4). From the chromatograms of the isolated fibers (charred elements in sherds), there are clear differences in thermal impact, which were also observed by tracking the B/T ratio (Figure 3, right graph). Some samples are dominated by MAHs and PAHs, and benzofuran, such as sample FT3 in Figure 4. Others have higher proportions of aliphatic compounds, such as FT1 and FT4, indicative of lower thermal impact.
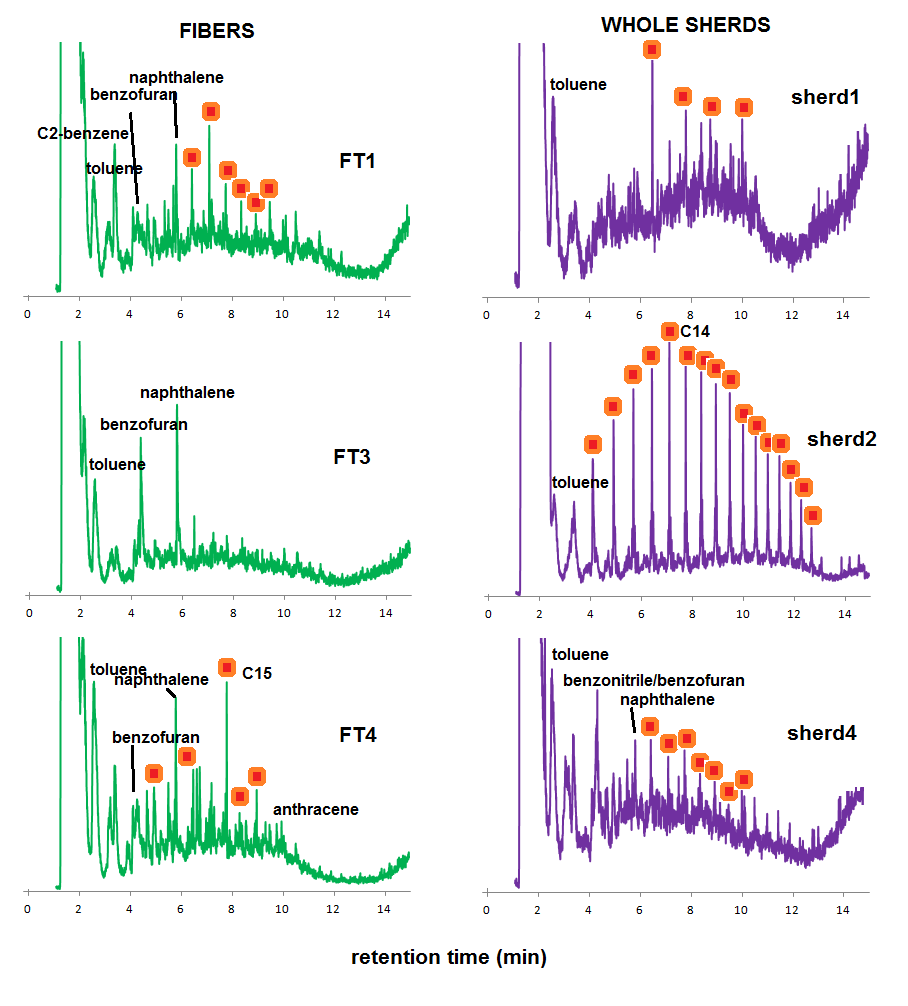
The chromatograms of whole sherds are more surprising. Sample sherd2, for example, shows a pyrolysis fingerprint that is strongly dominated by alkanes and alkenes, and with a chain length pattern that is clearly distinct as what was observed for the experimental chars or the isolated fibers from the archaeological sherds. This may be indicative of other organic ingredients (bitumen, dung or plant materials, for example) that were added to the ware before firing and were absorbed to it during its use. Sherd4 has a fingerprint that can be largely ascribed to Itla-okla remains.
Conclusions
Clearly, there are differences in firing intensity as observed from the balance between aliphatic products and phenols, vs. MAHs and PAHs. This became evident by comparing the Py-GC-MS fingerprints of the experimental thermosequences and the archaeological ware (isolated fibers). The range of temperatures are <500 and 500-600 ºCMFE. Note that these differences do not necessarily imply that there are large differences in the maximum temperature during firing: other factors such as the thickness of the material, and the duration of the process can explain the results as well. For example, relatively short firing or the use of thick ware may cause incomplete burn-off in the center of the ware where the organic matter may be least affected by the thermal alteration: such features can be recognized as sandwich morphologies of sherds (see figure below: dark core, lighter interior and exterior surfaces). The results of the whole sherd analysis may be indicative of other organic constituents and future studies will focus on the characterization of those materials.
References
Gilmore, Z.I., 2015. Direct radiocarbon dating of Spanish moss (Tillandsia usneoides) from early fiber-tempered pottery in the southeastern U.S. Journal of Archaeological Science 58, 1–8.
Kaal, J., Nierop, K.G.J., Martínez Cortizas, A., 2009. Characterisation of aged charcoal using a coil probe pyrolysis-GC/MS method optimised for Black Carbon. Journal of Analytical and Applied Pyrolysis 85, 408-416.
Kaal, J., Lantes Suárez, O., Martínez Cortizas, A, Prieto, B., Prieto Martínez, M.P., 2014. How useful is pyrolysis-GC-MS for the assessment of molecular properties of organic matter in archaeological pottery matrix? An exploratory case study from NW Spain. Archaeometry 56, 187-207.
Turney, C.S.M., Wheeler, D., Chivas, A.R., 2006. Carbon isotope fractionation in wood during carbonization. Geochimica et Cosmochimica Acta 70, 960–964.


